
Molecular control of the PD-1 inhibitory pathway
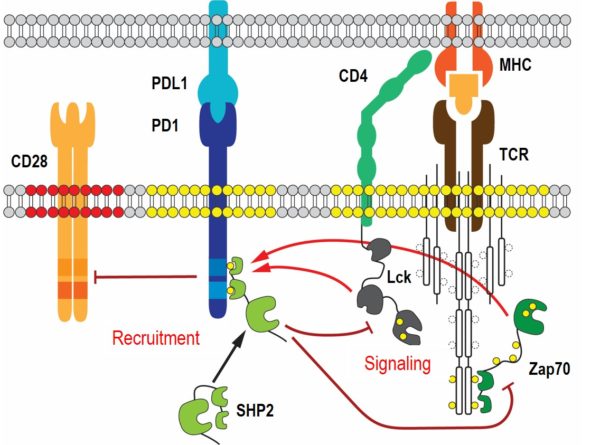
Upon T cell receptor (TCR) stimulation, excessive T cell activation is dampened by inhibitory receptors, such as PD-1, to prevent immunopathologies and autoimmunity. However, inhibitory signals can also prevent desired anti-tumor responses in patients. Targeting the inhibitory pathways with blocking antibodies, also known as checkpoint therapy, has been a major advance in cancer treatment. Sadly, checkpoint therapies have limited efficacies in the majority of cancer patients. The limited understanding of the molecular mechanisms downstream of inhibitory receptors has prevented the development of alternative approaches to current checkpoint therapies.

The main effector downstream of PD-1 is the tyrosine phosphatase SHP2. Recruitment of cytosolic SHP2 to ligand engaged PD-1 increases its catalytic activity and results in the dephosphorylation of many T cell signaling molecules, with the costimulatory receptor CD28 being a major target. Importantly, SHP2 is not solely utilized by the PD-1 pathway and has many opposing cellular functions in non-immune and immune cells. Proteomic analyses of SHP2 in our lab have identified several new phosphorylation sites that control SHP2’s catalytic activity and binding to PD-1. We are currently characterizing SHP2 mutants of the newly identified phosphorylation sites in vitro and in vivo using biochemical, biophysical and cellular approaches. State-of-the-art fluorescent microscopy, such as single molecule tracking and super-resolution microscopy, is applied to study the distributions, movements and interactions of wild-type and mutant SHP2 at the plasma membrane. Our goal is to elucidate molecular mechanisms that are unique to SHP2 downstream of PD-1 and to exploit them for alternative checkpoint therapies.