Modulating T cell signaling pathways for immunotherapies
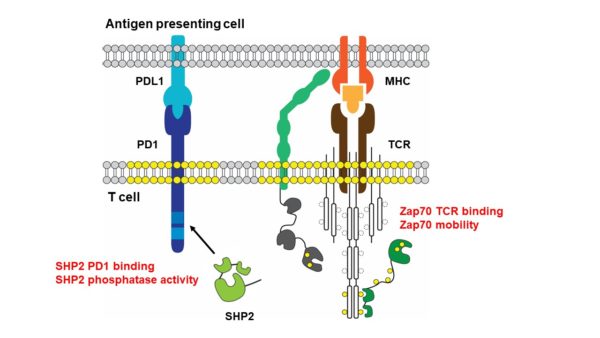
Immunotherapies that enhance the anti-tumor activity of T cells are the most promising cancer treatments to date. Generally, T cell-based immunotherapies rely either on augmentation of pre-existing T cell responses through reduction of inhibitory signals (checkpoint therapy), or adoptive transfer of T cells expressing receptors specific for tumor antigens. However, the efficacy of these methods is limited by the absence of endogenous responses, as well as low expression levels and/or low affinities of tumor antigens. We are developing new strategies based on reducing T cell activation thresholds or inhibitory signals by modulating assembly kinetics of the activating T cell receptor (TCR) or inhibitory PD-1 pathways, respectively. For this, we are focusing on the tyrosine kinase Zap70 (TCR pathway) and phosphatase SHP2 (PD1 pathway) as future therapeutic targets:
(1) Peptide MHC binding to the TCR results in its phosphorylation and thereby Zap70 recruitment. Zap70 becomes activated and transduces stimuli to downstream signaling molecules. Through multidisciplinary approaches, we have discovered that the cellular activity of Zap70 is tightly controlled by its TCR interaction dynamics and mobility at the plasma membrane.
(2) PD-1 ligand binding results in the phosphorylation of its cytosolic domain and subsequent recruitment and activation of SHP2. T cell inhibition relies on the dephosphorylation of T cell signaling molecules by SHP2. We have discovered that SHP2 activity and PD-1 binding is regulated through phosphorylation on multiple sites.
Based on our discoveries, we are testing if changing Zap70 and SHP2 conformation and receptor assembly kinetics have the potential to improve T cell responses in vivo, specifically against tumors. The laboratory also develops fluorescent probes that indicate the conformational states or receptor binding capacity of Zap70 and SHP2 to screen for small molecule inhibitors with the potential to treat cancer or autoimmunity.