T cell receptor signaling in space and time
T cells play critical roles during infections, cancer and autoimmunity. T cell activation is controlled by the T cell receptor (TCR), which recognizes peptides bound to MHC molecules on antigen presenting cells. TCR engagement results in assembly, phosphorylation and activation of its downstream signaling pathway at the plasma membrane. Our lab studies novel signaling principles that utilize the heterogenous and dynamic nature of the plasma membrane to control T cell activity and function. Our ultimate goal is to exploit these principles for future immunotherapies against diseases such as cancer and autoimmunity.

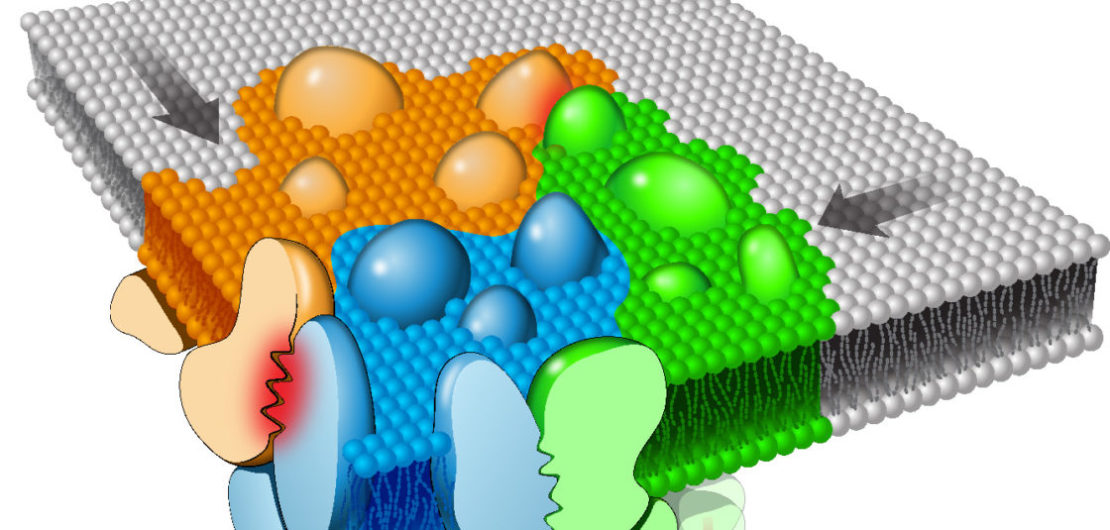
We have discovered that in quiescent T cells signaling molecules, i.e. the TCR and the linker of activation of T cells (Lat), are pre-clustered and segregated into nanometer scale membrane domains, termed protein islands or nanoclusters. Upon TCR engagement, nanoclusters concatenate into larger structures called microclusters without fusion or mixing of their content. Microcluster formation enables molecules, such as the kinase Zap70, to transfer signals between adjacent nanoclusters. In the case of Zap70, this is accomplished by a novel “Catch-And-Release” mechanism, a cycle of recruitment to the TCR, Zap70 activation, and release from TCR. This mechanism disperses signals within the plasma membrane and amplifies antigenic stimuli.
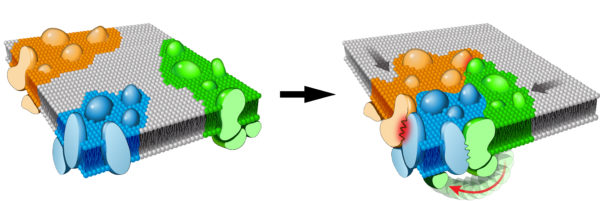
We are currently investigating additional molecular mechanisms that control TCR pathway assembly and signal propagation within the dynamic plasma membrane environment using cutting-edge microscopy as well as biochemical, biophysical and cell biological approaches.